

Charcoal Water Purifying Experiment
Sharing is caring!

Do you use some sort of charcoal water filter in your home? You might have a pitcher with a charcoal filter r or a filter that attaches to your faucet . Have you ever wondered how that process actually works? Try this charcoal water purifying experiment to learn all about it!
Science Experiments For Independent Learning
From start to finish, she is responsible for the experiment. Of course, she can ask questions or get help if she needs it, but I have found that the more she does the experiments, the less help she needs.
During the experiment, my daughter keeps notes on an experiment sheet. The rest of this blog post is her lab report as recorded on her experiment worksheet.
This charcoal water purifying experiment is a great experiment for middle school students to try on their own.
Can Charcoal Remove Molecules From Water?

My pre-experiment hypothesis is yes, charcoal can remove molecules from water. This is based on my knowledge of using charcoal filters to clean water.
- a measuring cup
- 2 baby food jars with lids
- red food coloring
Fill a measuring cup with 1/2 cup of water. Add 8 drops of red food coloring to the water and stir until mixed. Now, add 1/4 cup of colored water to each of the baby food jars.
To one jar, add 2 teaspoons of the activated carbon. Put the lids on both jars and set the jars in a place where they won’t be disturbed for a couple days.
Note the color of the jars when the carbon was added, 4 hours later, 24 hours later, and each day for 3 days.
Observations and Results
At the beginning of the experiment, the color of the water in the jars was the same.

By the next day, the water was almost totally clear in the activated carbon jar and the other jar was just as red as the beginning. On the second day after the experiment began, the water in the activated carbon jar was totally clear.
My results conclude that my hypothesis was correct. Charcoal can remove molecules from the water.
What Happened?
Activated charcoal is charcoal that has been specially treated to create cracks and holes in the charcoal. This treatment process is called activation and involves heating the charcoal to a high temperature. The high temperature changes the structure of the charcoal and makes it more porous and causes cracking. The cracking creates a greater surface area and more bonding sites. It is these binding sites that attract many chemicals in liquid and chemical form.
The food coloring is made of molecules. These molecules are bigger than water molecules.
When the food coloring, water, and charcoal are mixed together, the food coloring molecules break their bond to the water and attach to the carbon molecules of the activated carbon.
When the food coloring molecules (a liquid) “unstick” from the water (another liquid) and attach to the activated carbon (a solid), this is called adsorption. Adsorption happens when a liquid molecule separates from another liquid molecule and attaches to the molecule of a solid.
This is how the activated charcoal is able to remove the food coloring from the water. The food coloring molecules bind with the charcoal and come out of the water solution. This property of activated charcoal is what makes it a great filter/purifier.
Try this at home with your kids or let them try it for themselves!
For More Chemistry Activities
Check out The Homeschool Scientist’s Chemistry Resource Page!
Check out our printable unit on The Periodic Table of the Elements
Studying the properties of water? Check out these activities that demonstrate polarity, adhesion, cohesion, and more .

I hold a master’s degree in child development and early education and am working on a post-baccalaureate in biology. I spent 15 years working for a biotechnology company developing IT systems in DNA testing laboratories across the US. I taught K4 in a private school, homeschooled my children, and have taught on the mission field in southern Asia. For 4 years, I served on our state’s FIRST Lego League tournament Board and served as the Judging Director. I own thehomeschoolscientist and also write a regular science column for Homeschooling Today Magazine. You’ll also find my writings on the CTCMath blog. Through this site, I have authored over 50 math and science resources.
Your browser is not supported
Sorry but it looks as if your browser is out of date. To get the best experience using our site we recommend that you upgrade or switch browsers.
Find a solution
- Skip to main content
- Skip to navigation

- Back to parent navigation item
- Primary teacher
- Secondary/FE teacher
- Early career or student teacher
- Higher education
- Curriculum support
- Literacy in science teaching
- Periodic table
- Interactive periodic table
- Climate change and sustainability
- Resources shop
- Collections
- Remote teaching support
- Starters for ten
- Screen experiments
- Assessment for learning
- Microscale chemistry
- Faces of chemistry
- Classic chemistry experiments
- Nuffield practical collection
- Anecdotes for chemistry teachers
- On this day in chemistry
- Global experiments
- PhET interactive simulations
- Chemistry vignettes
- Context and problem based learning
- Journal of the month
- Chemistry and art
- Art analysis
- Pigments and colours
- Ancient art: today's technology
- Psychology and art theory
- Art and archaeology
- Artists as chemists
- The physics of restoration and conservation
- Ancient Egyptian art
- Ancient Greek art
- Ancient Roman art
- Classic chemistry demonstrations
- In search of solutions
- In search of more solutions
- Creative problem-solving in chemistry
- Solar spark
- Chemistry for non-specialists
- Health and safety in higher education
- Analytical chemistry introductions
- Exhibition chemistry
- Introductory maths for higher education
- Commercial skills for chemists
- Kitchen chemistry
- Journals how to guides
- Chemistry in health
- Chemistry in sport
- Chemistry in your cupboard
- Chocolate chemistry
- Adnoddau addysgu cemeg Cymraeg
- The chemistry of fireworks
- Festive chemistry
- Education in Chemistry
- Teach Chemistry
- On-demand online
- Live online
- Selected PD articles
- PD for primary teachers
- PD for secondary teachers
- What we offer
- Chartered Science Teacher (CSciTeach)
- Teacher mentoring
- UK Chemistry Olympiad
- Who can enter?
- How does it work?
- Resources and past papers
- Top of the Bench
- Schools' Analyst
- Regional support
- Education coordinators
- RSC Yusuf Hamied Inspirational Science Programme
- Science Education Policy Alliance
- RSC Education News
- Supporting teacher training
- Interest groups

- More navigation items
Carbon filtration and activated charcoal
In association with Nuffield Foundation
- No comments
Try this practical to remove objectionable tastes and odours from water using carbon in the form of activated charcoal
Carbon that has undergone special treatment (sometimes called activated charcoal) has useful absorption properties, capable of removing coloured and volatile material from mixtures. In this experiment, students use activated charcoal to tackle unwanted colours and odours in water, created by the addition of ink or food colouring and malt vinegar.
The experiment reflects the wide application of activated charcoal in organic chemistry and industry, including its use for the removal of objectionable tastes and odours from drinking water or medicines.
The practical is conveniently carried by groups of two or three and will take about 45 minutes.
- Eye protection
- Beaker, 100 cm 3
- Filter funnel
- Filter paper
- Test tubes, x2
- Test tube rack
- Activated charcoal, ten spatulas full
- Ink or food colouring, one drop (see note 5 below)
- Malt vinegar, 100 cm 3 (see note 6)
Health, safety and technical notes
- Read our standard health and safety guidance.
- Wear eye protection throughout.
- Activated charcoal, C(s) – see CLEAPSS Hazcard HC021 .
- Malt vinegar – see CLEAPSS Hazcard HC038a .
- Fountain pen ink (‘washable’ variety) is the best type of ink to use. A dilute solution of potassium permanganate(VII) could be used instead of ink or food flavouring.
- Juice from sauerkraut or pickles could be used instead of malt vinegar.
- Fold a piece of filter paper, place it in a funnel, and put the stem of the funnel into a test tube in a test tube rack.
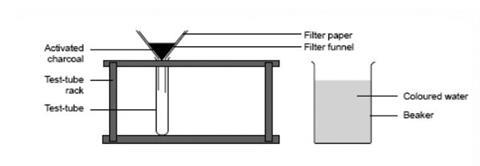
Source: Royal Society of Chemistry
How to set up the equipment before using activated charcoal to remove unwanted colour from water
- Add about five spatulas of activated charcoal to the funnel.
- Add one drop of ink or food colouring to 100 cm 3 of water in a beaker.
- Carefully pour some of the coloured water on to the charcoal in the filter paper. Note whether the drops of liquid in the test tube have lost the original colour.
- Repeat the activity with another test tube, this time pour 100 cm 3 of malt vinegar through the charcoal. Note whether the filtered liquid has lost some of its original strong smell.
Teaching notes
Students need to be warned that activated charcoal powder is extremely messy and difficult to remove from clothing.
The vinegar still smells after filtration, but noticeably less so.
Background theory
Heating wood to a very high temperature in the absence of air makes charcoal. When it is heated to an even higher temperature – about 930 °C – impurities are driven from its surface and it becomes ‘activated charcoal’. This activated charcoal can remove impurities in either the gaseous or liquid state from many solutions. It does so by the process of adsorption, by attracting these molecules to the surface of the charcoal.
Adsorption by charcoal is also used to remove unburned hydrocarbons from car exhausts, harmful gases from the air, and unwanted colours from certain products.
Students may find the difference between adsorption and absorption confusing. Adsorption is a process in which a gas, liquid, or a dissolved substance is gathered on the surface of another substance – eg charcoal. Absorption is a process in which a liquid is soaked up, as with blotting paper. It is taken in completely and mixes with the absorbing material – eg absorbent cotton.
Additional information
This is a resource from the Practical Chemistry project , developed by the Nuffield Foundation and the Royal Society of Chemistry. This collection of over 200 practical activities demonstrates a wide range of chemical concepts and processes. Each activity contains comprehensive information for teachers and technicians, including full technical notes and step-by-step procedures. Practical Chemistry activities accompany Practical Physics and Practical Biology .
© Nuffield Foundation and the Royal Society of Chemistry
- 11-14 years
- 14-16 years
- Practical experiments
Related articles

Solubility | Review my learning worksheets | 14–16 years
By Lyn Nicholls
Identify learning gaps and misconceptions with this set of worksheets offering three levels of support

How to teach chromatography at post-16
2024-03-11T04:00:00Z By Andy Markwick
Everything you need to help your students master the fundamentals of this analytical technique

Chromatography challenge | 16–18 years
By Andy Markwick
Explore analytical techniques and their applications with a chromatography investigation and research activity
No comments yet
Only registered users can comment on this article., more experiments.

‘Gold’ coins on a microscale | 14–16 years
By Dorothy Warren and Sandrine Bouchelkia
Practical experiment where learners produce ‘gold’ coins by electroplating a copper coin with zinc, includes follow-up worksheet

Practical potions microscale | 11–14 years
By Kirsty Patterson Four out of five
Observe chemical changes in this microscale experiment with a spooky twist.

Antibacterial properties of the halogens | 14–18 years
By Kristy Turner
Use this practical to investigate how solutions of the halogens inhibit the growth of bacteria and which is most effective
- Contributors
- Email alerts
Site powered by Webvision Cloud
Make a water filter
This fun science experiment turns dirty water clean. (Kind of.)
How do you clean up dirty water?
Not with soap! You need a filter, a device that removes impurities, like dirt, from water. The filter you’ll make here—with the help of an adult—is a super strainer, and it’ll help you clean up your act.
Ask a grown-up to cut the bottle in half. Then flip the bottle's top half over and put it in the bottom, so the top looks like a funnel. You'll build your filter in the top part.
Place the coffee filter (or bandanna, sock, etc.) at the bottom of your filter.
Add cotton balls, charcoal, gravel, sand, and / or other materials in layers. You can use just one of them or all of them. Tip: Think about which order to add them. Bigger filter materials usually catch bigger impurities.
Write down which filter materials you used and in what order you layered them.
Stir your dirty water and measure out a cup of it.
Get your timer ready!
Pour a cup of dirty water into your filter. Start the timer as soon as you begin pouring.
Time how long it takes for all the water to go through the filter. Then write down how long it took.
Carefully scoop out the filter materials, one layer at a time. What did each layer take out of the water?
Experiment! Clean the bottle and try again. Put the filter materials in a different order each time, and time each experiment. What do you discover?
WHAT'S GOING ON?
The slower, the better! The longer it takes for water to move through a filter, the cleaner it gets. Water slips easily through the filter materials, but bigger gunk, like dirt, gets trapped. The filter materials usually get finer and finer, so they can catch whatever was missed earlier. Activated charcoal can be near the end of the water’s path, because it uses an electrical charge to grab particles too small for us to see.
Your filtered water is not clean enough to drink. But a plant will love it!
Photographs by Mark Thiessen / NG Staff: Adapted from the Nat Geo Kids book How Things Work , by T.J. Resler
(AD) How Things Work: Then and Now
How things work, (ad) how things work: inside out, (ad) get the book.
- Terms of Use
- Privacy Policy
- Your California Privacy Rights
- Children's Online Privacy Policy
- Interest-Based Ads
- About Nielsen Measurement
- Do Not Sell My Info
- National Geographic
- National Geographic Education
- Shop Nat Geo
- Customer Service
- Manage Your Subscription
Copyright © 1996-2015 National Geographic Society Copyright © 2015-2025 National Geographic Partners, LLC. All rights reserved
Water Purification Through Activated Carbon (Project)

Introduction: Water Purification Through Activated Carbon (Project)
Many people around the world have limited access to water sources. This project is an inexpensive and simple solution to help solve the clean water crisis in some third world countries.

Step 1: The Problem

Clean Water is not available to over a tenth of the world's population, this filter will create an inexpensive and reliable way to filter out harmful materials from unclean water.
Step 2: Hypothesis
If the water used is clean from parasites and harmful bacteria, then the water will be perfectly safe to drink after using the filter.
Step 3: Variables
Independent Variable: The cleanliness of the water.
Dependent Variable: The unsafe materials left in the water.
Controlled Variable: The amount of charcoal, sand and gravel used.
Step 4: Background Research
I used two main scientific journals, the New York Department of Health [1] and the Purdue University Journal [2]. I also used Medium.com [3] to gather the results for my experiment.
I also know that there are many ways to purify water such as distillation and the use of filters.
Step 5: Materials

(Photo sources, in order: [4], [5], [6], [7], [8], [9], [10]
Purified, filter grade sand
Activated Charcoal
Clean Gravel
A plastic water bottle (preferably larger than 800ml with a circumference bigger than 8 inches)
3 clean and sanitary pieces of cloth
Food Coloring
Water (can be either clean or unclean)
Cylinder (can be substituted with a cup or another bottle)
2 coffee filters
Step 6: Procedure
1. Cut open the bottom of a large plastic bottle.
2. Stuff the cloths into the water bottle top to avoid the fine materials from spilling out and to hold back the carbon when filtering the water
3. Place the three coffee filters at the bottom of the bottle
3. Start by pouring in activated charcoal over the coffee filter until it reaches a minimum of 6 centimeters in height.
4. Add the purified sand on top of it, until it reaches at least 5 centimeters in height.
5. Add the gravel until there is only about 5 centimeters left of the bottle
6. Start by pouring 500ml of dirty water into the cylinder
8. Pour the mixture into the filter and wait until all of the water passes through.
9. Record the results
Step 7: Data Table

Step 8: Patterns
I noticed one pattern, which was that the water hardness and the nitrate/nitrite is very high. After researching about water filters effects on water hardness and nitrate/nitrite, I understood that charcoal filters do not affect water hardness and nitrate/nitrite, which is why there is a very apparent increase in nitrite/nitrate and water hardness. Water hardness is not harmful, although the high levels of nitrite and nitrate do have very detrimental affects on the health of the drinker.
Step 9: Data, Data Analysis, and Results

[3],[3],[3]
(Due to the recent pandemic, I was unable to conduct the experiment since I limited myself from leaving the house and could not get materials like purified sand, gravel and activated charcoal.)
(Data Extracted from: https://medium.com/@tylercrews/diy-water-filtrati...)
For use in real life situations, The filter made the water clear of any apparent and noticeable pollutants, although it had a high level of nitrate/nitrite (50 ppm). To put into perspective, the maximum amount of nitrate/nitrite to be considered drinking water by the United States Food and Drug Administration is 10 ppm. The water after being run through the filter also had 8 times the permissible total hardness (400 ppm). The water had a PH of 8, meaning that is within the accepted range and had no lead, pestecides, and Chlorine. The color of the water was also yellow, showing some discoloration but it could be due to using foraged materials which do not meet the industry's standard.
Step 10: Evaluating the Experiment and Improving the Experiment
Although I have not been able to conduct the experiment, the results attained by Medium.com [3] did not show the amount of harmful bacteria in the water. The experiment also might have used unclean materials since it used foraged materials from the wilderness instead of buying it pure from industries, meaning that there could be foreign objects in the materials used. The purified water was also yellow meaning that some dirt has stayed in the water, but this problem could have been prevented if she used multiple finer cloths and coffee filters to help make the sediment and dirt not seep through. The water also had a lot of nitrate/nitrite, meaning that it could be very harmful, although nitrite and nitrate cannot be removed through carbon filters and boiling. The only way to remove nitrite and nitrate without access to semi permeable membranes (for reverse osmosis) or an ion exchange resin (for ion exchange) is by distilling [11]. Although the amount of the two compounds varies in different water sources meaning that it may work in real life applications without any nitrate, as the amount of it is dependant on the source it came from. For water sources with high amounts of bacteria, repetitive use of the filter could cause an increase of it in the purified water, so the best course of action is to pasteurize it [1] (boiling the water at 65 degrees Celsius at least for at least 20 minutes).
Step 11: Conclusion (Evaluating the Hypothesis)
In conclusion, we can deduce that the experiment did filter out many harmful substances, although the water is still unsafe to drink if the water source has high levels of nitrite/nitrate (50 ppm,
Step 12: Application (Why I Chose to Do This Project and What the Project Is About)

Over 790 million [12] people around the world do not have access to clean water. This project's purpose is to make a cheap and easily accessible water filter to help combat the lack of availability of clean water in poor countries. This filter helps remove most sediment and molecules that make water in such countries not safe to drink, and to further reduce the risk of harmful bacteria being in the water the water should be pasteurized at 65 degrees Celsius (or above) for 20 minutes or more [1]. This helps kill many different types of harmful organisms that are present in unclean water sources like Giardia, Cryptosporidium, Endameba, the eggs of certain worms, Vibrio cholera, Shigella, Salmonella bacteria, and the strains of bacteria that cause Typhoid [1].
Step 13: Bibiliography
(All photos used in the video are cited below)
[1] “Boil Water Response - Information for the Public Health Professional.” New York State Department Of Health, www.health.ny.gov/environmental/water/drinking/bo...
[2] Kamrin, Michael, et al. “Distillation For Home Water Treatment.” Purdue University, Michigan State University, www.extension.purdue.edu/extmedia/WQ/WQ-12.html.
[3] Crews, Tyler. “DIY Water Filtration Systems: Do They Really Work?” Medium, 21 Apr. 2017, medium.com/@tylercrews/diy-water-filtration-systems-do-they-really-work-5a22d2ccb8e2.
[4] “The Sand Purified Buy in Kaskelen.” All, kz.all.biz/en/the-sand-purified-g164839.
[5] Zarek, Jonnie. “Activated Charcoal.” MakeYourOwn.buzz, www.makeyourown.buzz/activated-charcoal/.
[6] Herold's Garden Center http://heroldsgardencenter.com/shop/product/34-cle...
[7] Drink More Custom Water https://www.drinkmorecustomwater.com/bottle-galler...
[8] Pinterest https://www.pinterest.cl/pin/680676931154375938/
[9] Drifton https://www.drifton.eu/shop/110-laboratory-glasswa...
[10] Amazon https://www.amazon.com/BUNN-12-Cup-Commercial-Filt...
[11] “Nitrates in Well Water.” FilterWater, 23 Dec. 2016, “Boil Water Response - Information for the Public Health Professional.” New York State Department Of Health, www.health.ny.gov/environmental/water/drinking/bo...
[12]“Global WASH Fast Facts.” Centers for Disease Control and Prevention , 11 Apr. 2016, www.cdc.gov/healthywater/global/wash_statistics.h...
[13] Bassmaster https://www.bassmaster.com/greg-hackney/catching-...
[14] Pinterest https://www.pinterest.com/pin/590323463627696112/
[15] Africa Rising https://www.africarising.tv/downloads/african-sto...
- For Teachers

- Everyday Activities
- Experiments
Water Filter
Can you make muddy water crystal clear?
Make your own water filter!
You'll be able to remove dirt, heavy metals and chemicals from filthy water!
Watch the video on YouTube: https://youtu.be/fHbeJaScUnA
You Will Need
2 liters of stream or river water (or mix a handful of dirt into 2 liters of water)
2 empty clear 1-liter plastic soda bottles
30 cotton balls
A thumb tack
A stick or skewer
Optional: 2 cloth circles, about 6 inches in diameter, or round coffee filters
2 cups of cleaned activated carbon (charcoal) (this can be found in most pharmacies in the vitamin section)
2 cups of sand
1 cup of fired clay pieces (we crushed up inexpensive terracotta flower pots)
Several empty 12 oz cups to collect your filtered water (The bottle should be able to sit snugly in the top of the cups)
Materials & Directions PDF
- Example: Does changing the arrangement of the layers affect how quickly the water is filtered?
- 1 hole per “bump” on the bottom of the bottle works well.

- Layer 10-15 cotton balls in the bottom of each bottle. Pull them slightly apart, then use the skewer or stick to smush them down into all of the bumps in the bottom of the bottle. The cotton needs to cover the entire bottom of the bottle to keep the sand from coming out.
- Getting the cloth or filter in the bottle and then over the cotton can be tricky. You could ask an adult to cut the spout off your bottle so you have a larger opening to work with.
- A sand layer of about 7cm or 2 ½ inches deep is recommended.
- Experiment by adding additional layers. You could make one filter using the charcoal, make another one using the fired clay, or make one filter that uses both, or make one with just sand - experiment!
- Place a cup under your filter to catch the filtered water.
- Place the small end of the funnel in the top of the bottle and pour about 1.5 cups of the dirty water into the top of your filter. Wait several minutes for the water to filter through. The water should drip SLOWLY out of the bottom of the filter.
- See how many times you need to pour the water through your filter(s) until it becomes clear. Make a chart to track which filter works best.
- DO NOT DRINK IT! (In many cases, boiling the water for at least 1 minute would make it safe to drink, but we DO NOT recommend drinking the water from this experiment, just to be safe!)
Discovery Questions
Beginning the experiment, during the experiment, after the experiment, how it works.
The different layers of the filter help to pull the dirty particles out of the water.
- The cotton ball layer helps to keep the other layers of your filter from falling out into your water.
- The sand layer acts as a coarse filter for large muddy particles and to keep the activated charcoal or clay particles from getting into the cleaned water.
- The fired ceramic clay attracts metallic ions and is good for attracting and filtering out metallic particles.
- The activated charcoal layer is amazing at trapping the impurities in its network of holes and tunnels.
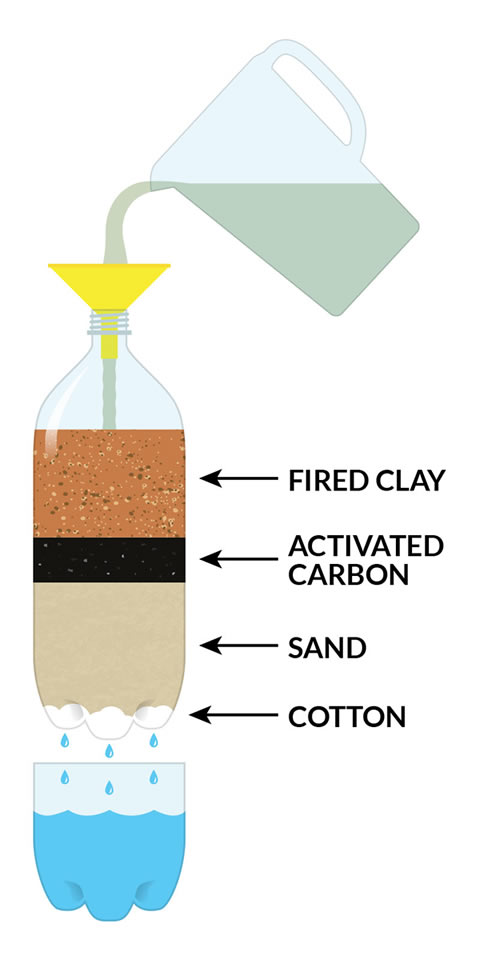
Activated charcoal is carbon that has been treated with oxygen at very high temperatures. The oxygen eats away at the carbon and makes all kinds of tunnels and pores. Just three grams of activated charcoal can have as much surface area as a football field! As water passes through this porous charcoal, the little particles and impurities get trapped inside the charcoal. “Activated” means it has a slightly positive charge and works like a magnet on negatively charged impurities that are attracted and bind to the outside of the charcoal.
After running your dirty water through the filter a number of times, it will appear to be nice and clean. But even though it looks completely clear, it MUST be thoroughly sterilized before it could be used for drinking. Boiling water is a common technique for removing pathogens, but avoid doing so unless absolutely necessary. (Again, we DO NOT recommend drinking the water from this experiment, just to be safe!)
Did you know that 75% of the earth’s surface is covered by water? Most of it is salt water and can’t be used for drinking. In fact, less than 1% of the earth’s water can be used by people!
As you saw with this experiment, filtering dirty water to make it clean enough to drink takes time and effort. It is very important to save the water we use every day and not waste it. What are some ways you can conserve water every day?
For more information, visit:
https://www.epa.gov/watersense/how-we-use-water

People often assume that activated charcoal is safe to consume whereas non-activated charcoal is not safe. This assumption is false. I have never found a single source that suggests non-activated charcoal is unsafe. According to many sources, the only difference between activated charcoal vs. non-activated charcoal is that activated charcoal is more effective at removing toxins from water, (and your body) , because it has been processed in a way that makes each granule have a larger surface area.
I like to question things. Just because something is common knowledge doesn’t always mean it is accurate knowledge!
In this post, I will show the results from my experiment where I tested activated charcoal vs. non-activated charcoal to see which would remove more chlorine from water.
***Note: “Charcoal” and “Carbon” may be used interchangeably.
Activated Charcoal Vs. Non-Activated Charcoal - An Experiment
Activated charcoal source.
The activated charcoal that I used for my experiment was sourced from a Brita filter cartridge. In the photo below you can see there are black specks and tan specks inside of the disassembled filter cartridge. The contents consist of “coconut-based activated carbon with ion exchange resin in a BPA-free housing to reduce chlorine taste and odor, zinc, and the health contaminants copper, cadmium, and mercury.” (The black specks are carbon granules and the tan specks are resin granules.)

Non-Activated Charcoal Source
For the non-activated charcoal source in this experiment, I burned some mesquite wood that I found on my property. Then, I crushed it and sifted it into a fine powder. To learn how I did it, click on this link: H ow to Make Non-Activated Charcoal Eye Shadow!

The Experiment
Will non-activated charcoal remove the same amount of chlorine as activated charcoal in tap water samples?
First, I placed the activated charcoal and non-activated charcoal in two separate metal mesh strainers. The main limitation of this experiment is that I didn’t weigh either of the charcoals as I didn’t have access to scales. Another limitation of the experiment is that the activated charcoal is mixed with resins as I mentioned previously.

Then, I dipped the activated charcoal and non-activated charcoal in two separate beakers filled with 200mL of chlorinated municipal water (tap water.) In addition, I set out a sample of the same volume with no charcoal. I did this because chlorine can evaporate from water on it’s own, without the help of a filter.
I let the samples sit for about 4 hours, so the charcoal/carbon could have time to pull chlorine from the water.

For the third step, I tested the chlorine levels of the water before I started the 4 hour timer on the above samples. Unfortunately, I forgot to grab a photo of the midnight reading, but it was within range (between 3.7mg/L to 4.0mg/L.) This is the amount of chlorine that many water plants require in tap water when sent out into distribution from the plant.
After the 3 samples sat for 4 hours I tested their chlorine levels. The results were shocking!
Unfiltered Water Vs. Activated Charcoal Water
As I said, my midnight chlorine readings were within range (between 3.75mg/L and 4.0mg/L.) The unfiltered sample reading on the left shows that some of the chlorine evaporated without any charcoal influence within a 4 hour period. The sample on the right shows that a great deal of chlorine was removed from the activated charcoal. However, the free ammonia spiked higher than the healthy drinking range!

Unfiltered Water Vs. Non-Activated Charcoal Water
This is the moment of truth… The moment that answers the question, “Is non-activated charcoal as effective at removing chlorine as activated charcoal?” According to my experiment – yes! Non-activated charcoal has about the same chlorine removal effectiveness as activated charcoal. This is exciting news!
Unfortunately, the non-activated charcoal also made the free ammonia levels spike too high for safe human consumption.
Interesting…

It’s exciting to discover that non-activated charcoal is as effective at removing chlorine as activated charcoal. Of course, there were some limitations to this experiment that could have lead to a bit of inaccuracy. More testing with more accurate measurements would be required to make a better conclusion.
If you’re wondering why the free ammonia spiked in the filtered samples, I have a simple explanation for that. Liquid ammonia sulfate is added to water in some water treatment systems because it combines with chlorine to create monochloramines. Monochloramines are considered a safer form of chlorine for human consumption. In addition, monochloramines have a longer lasting residual, meaning they can disinfect water at farther reaches of the distribution system, like the Crab Shack at the edge of town.
Thanks for Reading!
To learn more about why chlorine is added to water and how you can filter it out, Click here!
If you liked this post, please subscribe and share! I promise to never flood your inbox. I send emails about once a month max.
Share this:

Activated Carbon in Water Filtration

By: Stephanie Ph. Year: 2020 School: Talbert (Samuel E.) Middle School, 8th grade Division: Junior
The purpose of my experiment is to test out which plant fiber material would work best for making activated charcoal.
For my experiment, I will be testing different types of plant fibers to make charcoal, which will then be made into activated carbon. To test out the effectiveness of my activated carbon, I will use it in a water filter. The cleaner the activated carbon filtrates the water, the more effective it is.
The overall purpose is to filter water using a quick and easy way that won’t cost a lot of money. So, I started making charcoal from different bases. In my experiment, I used the bases, wood, coconut shells, and tangerine peels to make charcoal, and heated the bases until they turned into charcoal. I then added calcium chloride to enhance it’s absorption properties, and then heated it once more to activate it. I then made basic water filter swith the activated carbon at the bottom, and tested out the water filter using contaminated water.
In the end, the coconut shell base had the best result for purifying water, and tangerine peels were the least effective activated charcoal base. Although, the wood base was close behind the coconut shells and was also an efficient water filter. The tangerine peels weren’t effective at all, it only filtered some impurities out of the water, but didn’t do an effective job. For the coconuts hell base, it filtered out 40 total alkalinity out of contaminated water. In conclusion, the coconut shell base was the best base in activated carbon to filtrate water.
- Fair Awards
- Past Projects
- Registration
- Rules and Guidelines
- Project Categories
- Board and Sponsors
- Mission & Values
- Mentor Match Program
- Thermo Fisher Junior Innovators Challenge

IMAGES
COMMENTS
Activated charcoal is charcoal that has been specially treated to create cracks and holes in the charcoal. This treatment process is called activation and involves heating the charcoal to a high temperature.
Try this practical to remove objectionable tastes and odours from water using carbon in the form of activated charcoal. Includes kit list and safety instructions.
In this experiment, you will filter 3 different water samples with varying amounts of a “contaminant” (black ink) using 3 different types of filtration: granular activated carbon, powdered activated carbon, and no carbon.
Activated charcoal can be near the end of the water’s path, because it uses an electrical charge to grab particles too small for us to see. Your filtered water is not clean enough to drink. But...
Water Purification Through Activated Carbon (Project): [14] Many people around the world have limited access to water sources. This project is an inexpensive and simple solution to help solve the clean water crisis in some third world countries.
Activated charcoal is carbon that has been treated with oxygen at very high temperatures. The oxygen eats away at the carbon and makes all kinds of tunnels and pores. As water passes through this porous charcoal, the little particles and impurities get trapped inside the charcoal.
Apr 26, 2017 · Active charcoal is a good adsorbent, and depending on the technology of its manufacture, the surface of one gram of charcoal that can adsorb substances reaches...
That means that the material inside the filter is carbon, or a special form of carbon, called activated carbon. What makes activated carbon special is that it is a very porous form of carbon—almost like a sponge—that has many tiny microscopic pores that soak up water.
According to many sources, the only difference between activated charcoal vs. non-activated charcoal is that activated charcoal is more effective at removing toxins from water, (and your body), because it has been processed in a way that makes each granule have a larger surface area.
Jun 3, 2020 · The purpose of my experiment is to test out which plant fiber material would work best for making activated charcoal. For my experiment, I will be testing different types of plant fibers to make charcoal, which will then be made into activated carbon.